Carbon dioxide adsorbent / Soda lime
Model: XT-DES-01
Uses: Absorbing carbon dioxide CO2
Shape: Ф 2.5-3.5mm, length 4-7mm, columnar particles
Composition: calcium hydroxide, sodium hydroxide
Characteristic: high purity; high absorption rate; low resistance, uniform ventilation; low cost
Index: See product details for specific parameters
Package: 20kg plastic container or carton
Application area: widely used in oxygen-supply respirators and self-rescue devices to absorb carbon dioxide exhaled by the human body, as well as in environments such as chemical industry, machinery, electronics, industry and mining, medicine, and laboratories that need to
Product Details
1)Product introduction
Carbon dioxide adsorbent is also called calcium hydroxide, soda lime or soda lime. Its main components are calcium hydroxide, sodium hydroxide and water. The carbon dioxide adsorbent is pink or white columnar particles with a diameter of 3mm and a length of 4-7mm. Its structure is loose and porous, with large adsorption surface area and good permeability. The pink particles turn white after absorbing CO2, and the white particles turn purple after absorbing CO2. Carbon dioxide adsorbents have a very high absorption rate of carbon dioxide and can be widely used in oxygen-supply respirators and self-rescue devices to absorb carbon dioxide exhaled by the human body, as well as in environments such as chemical industry, machinery, electronics, industry and mining, medicine, and laboratories that need to absorb CO2.
2)Parameters
|
Ingredients |
Ca(OH)2, NaOH, H2O |
|
Shape |
White or Pink columnar |
|
Size |
Diameter:3mm Length: 4-7mm |
|
Absorptivity |
≥33% |
|
Moisture |
12% |
|
Dust |
< 2% |
|
Service life |
2 years |
3)Product advantages
A)High purity. The carbon dioxide adsorbent produced by Xintan does not contain any impurities.
B)High absorption rate. Carbon dioxide adsorbent have a large specific surface area and can fully absorb CO2 gas exhaled by the human body.
C)Low resistance, uniform ventilation. The main component of carbon dioxide adsorbent is calcium hydroxide, and its structure is loose and porous, which is conducive to the full absorption of carbon dioxide gas inside the adsorbent and the reduction of ventilation resistance.
D)Low cost. The raw material calcium hydroxide used in carbon dioxide adsorbent is more than 85%, which can not only significantly improve the adsorption rate of carbon dioxide, but also effectively reduce the use of commonly used raw materials sodium hydroxide and potassium hydroxide, and reduce product costs.
4)Applications
Carbon dioxide adsorbent is widely used in coal mine underground rescue cabins and refuge chambers to absorb carbon dioxide exhaled by the human body. It is also suitable for positive pressure oxygen respirators, isolation oxygen respirators and self-rescuers, as well as in aerospace, submarines, diving, chemical industry, Machinery, electronics, industry and mining, medicine, laboratories and other environments that need to absorb carbon dioxide.

5)Remark:
Seal in a dry place and store away from light. Warehouse temperature: 0-40℃.

Qualification Certificate

Test Report

Quality Management System Certificate

SGS
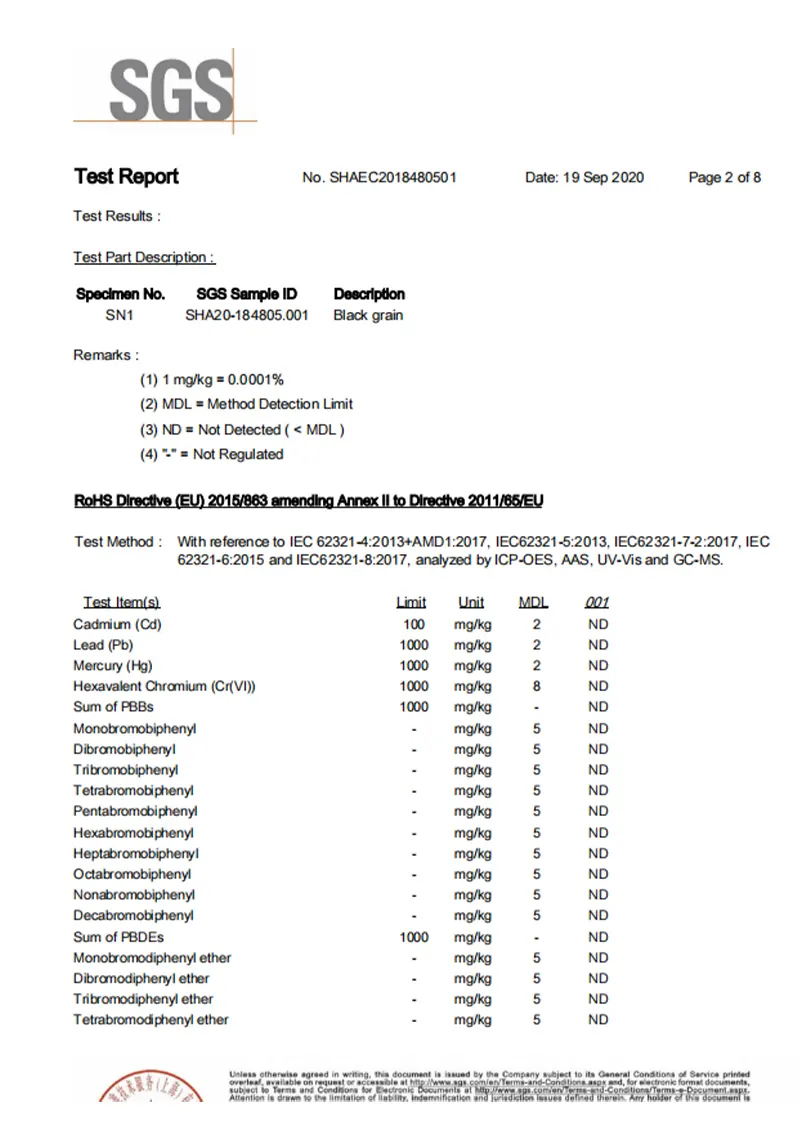
SGS
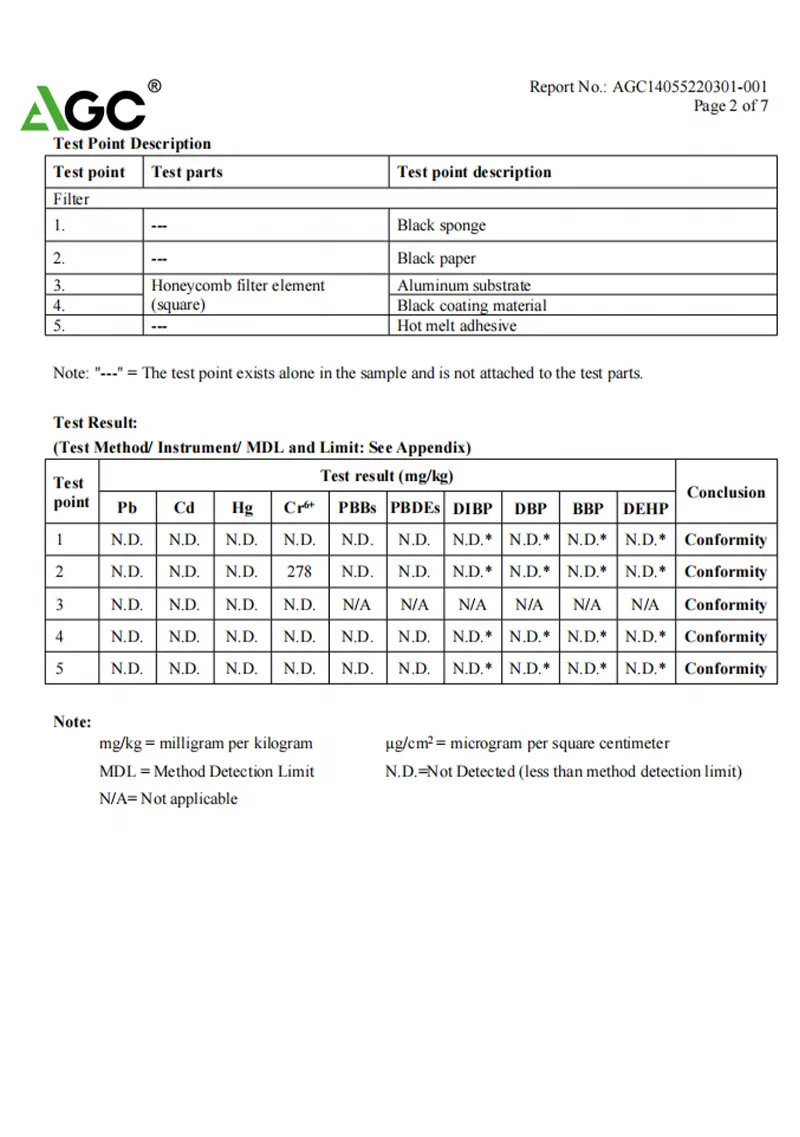
AGC
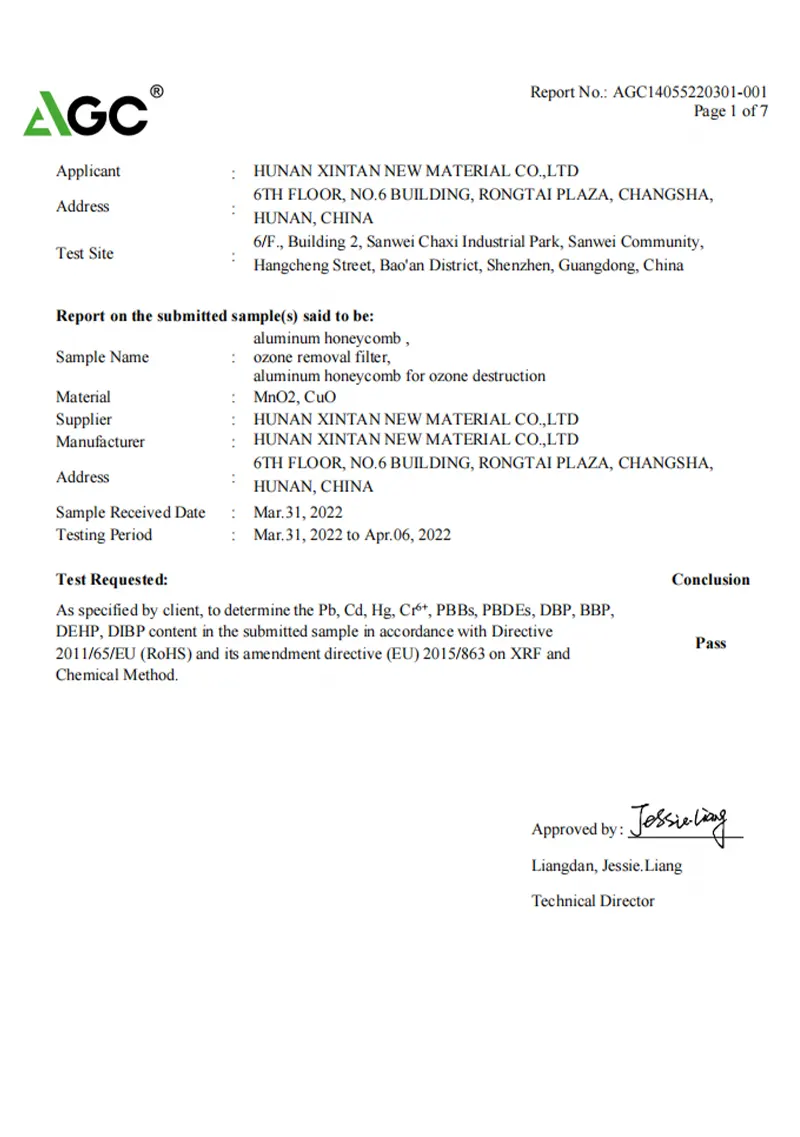
AGC
Related Products
Carbon molecular sieve, which are composed of elemental carbon, are a new type of adsorbent and an excellent non-polar carbon material. Hunan Xintan carbon molecular sieve is black columnar particles with a diameter of 1.0-1.3mm or customized. The product has the characteristics of strong nitrogen production capacity, high nitrogen recovery rate, long service life and low production cost. Carbon molecular sieve contain a large number of micropores, which have a strong instantaneous affinity for oxygen molecules and can be used to separate O2 and N2 in the air. In industry, carbon molecular sieves are often used in pressure swing adsorption (PSA) nitrogen production, which is suitable for various types of pressure swing adsorption nitrogen generators.
CO removal catalyst (Ceramic honeycomb)
CO removal catalyst (Honeycomb) uses cordierite honeycomb ceramic as a carrier and is a highly active catalyst prepared from a variety of rare earth metals. The CO removal catalyst (Honeycomb) produced by Hunan Xintan has the characteristics of high structural strength, strong surface coating adhesion, and is not easy to fall off. This catalyst can not only be used to treat carbon monoxide in flue gas, but also has high catalytic performance in chlorine-containing working conditions, and can be used for the treatment of chlorine-containing organic waste gas, and is widely used in the treatment ofsteel flue gas, lime kiln flue gas, waste incineration smoke and other smoke.
CO removal catalyst (Pt and Pd)
CO removal catalyst (pellet with Pt and Pd) is a precious metal palladium catalyst with alumina as a carrier. The carbon monoxide precious metal catalyst produced by Hunan Xintan is dark brown spherical particles with a diameter of 3-5mm, which is mainly used to remove H2 and CO at 20℃~300℃. The catalyst can convert CO to CO2 and H2 to H2O. It does not contain MnO2, CuO or S, so it can be safely used for CO purification in CO2. CO removal catalyst (pellet with Pt and Pd) have the characteristics of high catalytic efficiency, stable performance, safety, no energy consumption and long service life, and are widely used in the food industry.
CuO catalyst (for removing oxygen from Nitrogen or Methanol Synthesis)
CuO catalyst (for removing oxygen from Nitrogen or Methanol Synthesis) developed by Hunan Xintan is made of a high proportion of copper oxide (CuO) and inert metal oxides. It is a black cylindrical particle with a diameter of 5mm and a length of 5mm. The catalyst has the characteristics of high active component content, high packing density, low cost and long service life. It is a catalyst used to remove oxygen from nitrogen or inert gases such as helium and argon, which can efficiently convert oxygen to CuO without the need for additional energy. It does not contain any dangerous substances. The catalyst is widely used in gas treatment because of its high efficiency. The catalytic deoxidation reaction equation is as follows: CuO+H2=Cu+H2O 2Cu+O2=2CuO










